Our Aim: Chronic Cough Study
You might qualify for participation in a clinical study. If eligible, you could receive*:
Study-related care from local doctors at no cost
Study drug at no cost
compensation upon qualification
There's no obligation, so check now to see if you meet the criteria.
About the Chronic Cough Study?
Are you struggling with a persistent cough that just won't go away? You might qualify for the Chronic Cough Studies. We're looking for individuals aged 18 to 80 who have experienced a chronic cough for at least a year, despite trying various medical evaluations and medications.
We welcome participants of all genders, ages, and ethnicities to join our studies. By taking part, you can contribute to groundbreaking research aimed at providing relief for those dealing with chronic cough. Seize this opportunity to make a meaningful impact!
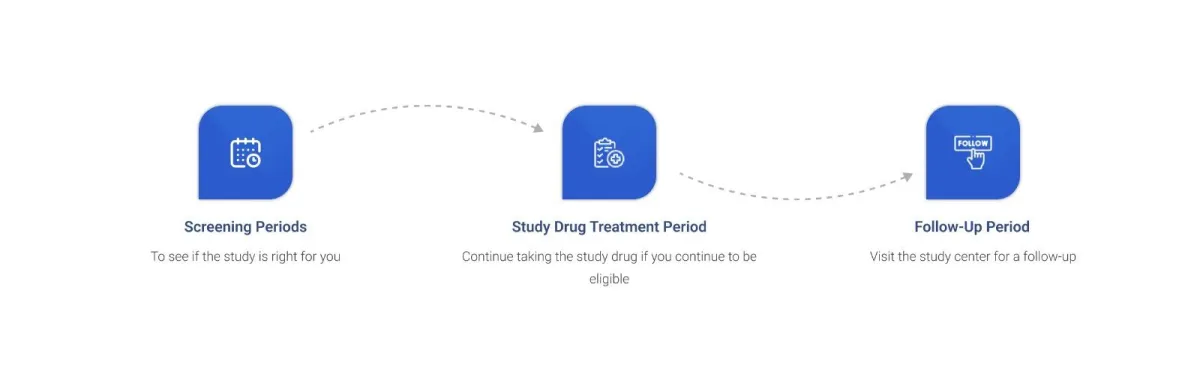
Who can Participate?
*If you are between 18 and 80 years of age
*Have had a cough for at least 1 year that did not improve or only partially improved following medical evaluation and prescribed or over-the-counter medications.
We are looking for participants from a variety of backgrounds (gender, age, and ethnicity).
If you or someone you love is suffering from Chronic Cough, we invite you to see if you qualify to take part in a clinical research study that will help the delay and treatment of symptoms associated with these diseases.
Sign Up
What happens if I Sign Up? We will match you to a study site in your area that needs participants with Chronic Cough or notify you when one becomes available. The study team will then contact you and you may have the opportunity to participate if qualified.
If you think you might like to participate in the Chronic Cough Study or would like more information, please enter your information below so we can see if you may qualify and can contact you about the study. Keep in mind that participation is entirely voluntary. If you do decide to take part in the study, you may change your mind about participating at any time.
Fields marked with an * are required.
I confirm that by submitting I'm agreeing to be contacted by the My Recruitment Hero LLC and affiliated Research Institutes to contact for this and future similar studies.

Frequetly Asked Question
What is a clinical research study?
A Clinical Research Study (also called a clinical trial) is a medical study that helps answer important questions about an investigational drug or device, such as: does it work, or how effective is it compared to another drug/device?All medications must be tested in clinical research studies before they can be approved by regulatory authorities for doctors to prescribe to patients. Without people taking part in these studies, we would have no new treatments. The volunteers who participate in clinical research studies play a major role in helping to advance medicine.
What happens to personal information?
The study team respects and protects the participant’s privacy. The study team will not share the participant’s information except as required by law. The study team will store the participant’s personal information with codes that do not identify the participant. The Informed Consent Form has more information about the participant’s privacy.
Can participation in the study stop at any time?
Yes, your involvement in the study is voluntary. The participant may stop participating at any time. If the participant decides to stop early, please notify the study team. The study team will ask the participant to return to the study office at least once for follow up tests to check the participant's health once the investigational medication has been stopped.